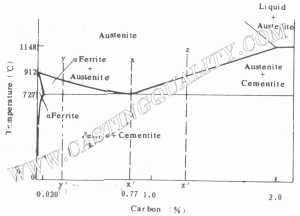
Steels having less than the eutectioid amount of carbon (less than 0.77%) are known as hypoeutectoid steels. Consider now the transfomation of such a material represented by cooling along line y – y’ in Fig 1.1. At high temperatures, the material is entirely austenite, but upon cooling enters a region where the stable phases are ferrite and austenite. Tie-line and lever-law calculations show that low carbon ferrite nucleates and grows, leaving the remaining austenite richerin carbon. At 727 ‘C (1341 F), the austenite is of eutectoid composition (0.77% carbon) and further cooling transforms the remaining austenite to pearlite. The resulting structure is a mixture of primary or proeutectoid ferrite (ferrite that formed above the eutectoid reaction) and regions of pearlite.
Hypereutectoid steels are stees that contain greater than the eutectoid amount of carbon. When such a steel cools, as in z – z’ of Fig.1.1 the process is similar to the hypoeutectoid case, except that the primary or proeutectoid phase is now cementite instead of ferrite. As the carbon rich phaser forms, the remaining austenite decreases in carbon content, reaching the eutectoid composition at 727 ‘C (1341F). As before, any remaining austenite transforms to pearlite upon slow cooling through this temperature.
It should be remembered that the transitions that have been descibed by the phase diagrams are for equilibrium conditions, which can be approximated by slow cooling. With slow heating, these transitions occur in the reverse manner. However, when alloys are cooled rapidly, entirely different results may be obtained, because sufficient time is not provided for the normal phase reactions to occur, in such cases, the phase diagram is no longer a useful cool for engineering analysis.