The understanding of heat tratment is embraced by the broader study of metallurgy. Metallurgy is the physics, chemistry, and engineering related to metals from ore extraction to the final product. Heat treatment is the operation of heating and cooling a metal in its solid state to change its physical properties. According to the procedure used, steel can be hardened to resist cutting action and abrasion, or it can be softened to permit machining. With the proper heat tratment internal stresses may be removed, grain size reduced, toughness increased, or a hard surface produced on a ductile interior. The analysis of the steel must be known because small percentages of certain elements, notably carbon, greatly affect the physical properties.
Alloy steels owe their properties to the presence of one or more elements other than carbon, namely nickel, chromium, manganese, molybdenum, tungsten, silicon, vanadium and copper. Because of their improved physical properties they are used commercially in many ways not possible with carbon steels.
The following discussion applies principally to the heat treatment of ordinary commercial steel known as plain-carbon steels. with the process the rate of cooling is the controlling factor, repid cooling from above the critical range results in hard structure, whereas very slow cooling produces the opposite effect.
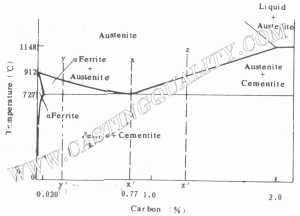
A Simplified Iron – Carbon Diagram
If we focus only on the materials normally known as steels, a simplified diagram is often used. Those portions of the iron-carbon diagram near the delta region and those above 2% carbon content are of little importance to the engineer and are deleted. A simplified diagram, such as the one in fig. 1.1 focuses on the eutectoid region and is quite useful in understanding the properties and processing of steel.
The key transition described in this diagram is the decomposition of single phase austenite (y) to the two phase ferrite plas carbide structure as temperature drops. Control of this reaction, which arises due to the drastically different carbon solubilities of austenite and ferrite, enables a wide range of properties to be achieved through heat treatment.
To begin to understand these processes, consider a steel of the eutectoid eomposition, 0.77% carbon, being slow cooled along line x-x’ in Fig.1.1. At the upper temperatures, only austenite is present, the 0.77% carbon being dissolved in solid solution with the iron. when the steel cools to 727 ‘C(1341 F), several changes occur simultaneously. The iron wants to change from the fcc austenite structure to the bcc ferrite structure, but the ferrite can only contain 0.02% carbon in solid solution. the rejected carbon forms the carbon rich cementite intermetallic with composition Fe3C. In essence, the net reaction at the eutectoid is
austenite (0.77%C) ——- ferrite (0.02%C) + cementite (6.67%C)
Since this chemical separation of the carbon component occurs entirely in the solid state, the resulting structure is a fine mechanical mixture of ferrite and cementite. Speciments prepared by polishing and etching in a week solution of nitric acid and alcohol reveal the lamellar structure of alternating plates that forms on slow cooling. this structure is composed of two distinct phases, but has its own set of characteristic properties and goes by the name pearlite, because of its resem blance to mother of pearl at low magnification.
To Be Continued…………